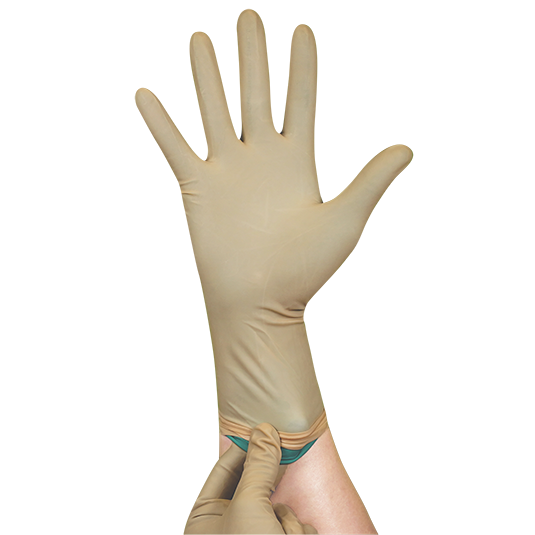
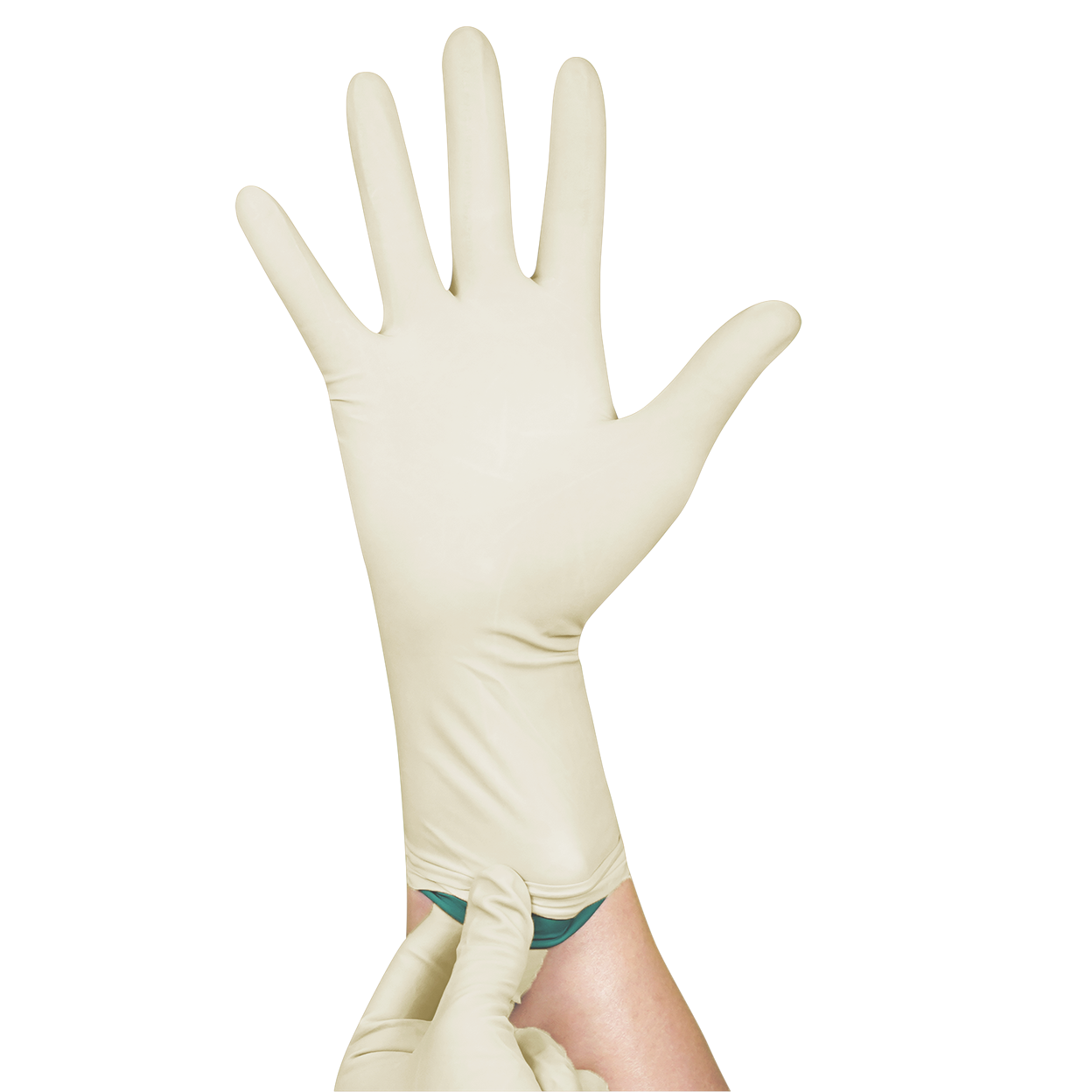
Double Donning Sterile Latex Surgical Powder Free Glove
Double Gloves provide higher protection during intensive surgeries and double assurance to avoid cross-infection between users and patients. Contrast and bright inner colored gloves ease the detection of expose barrier penetration or puncture. Fully anatomical design with curved fingers follow the natural finger shapes at rest provides free range motion and minimises hand fatigue during surgical procedure. Palm textured surface enhances slip resistance for extra grip and control. Polymer coated powder free interior enables incredible smooth donning experience.
Colour