Sterile Nitrile Surgical Powder Free Glove
Excellent alternative which eliminates protein allergic reaction and suitable for those who are sensitive to natural rubber latex.
Colour
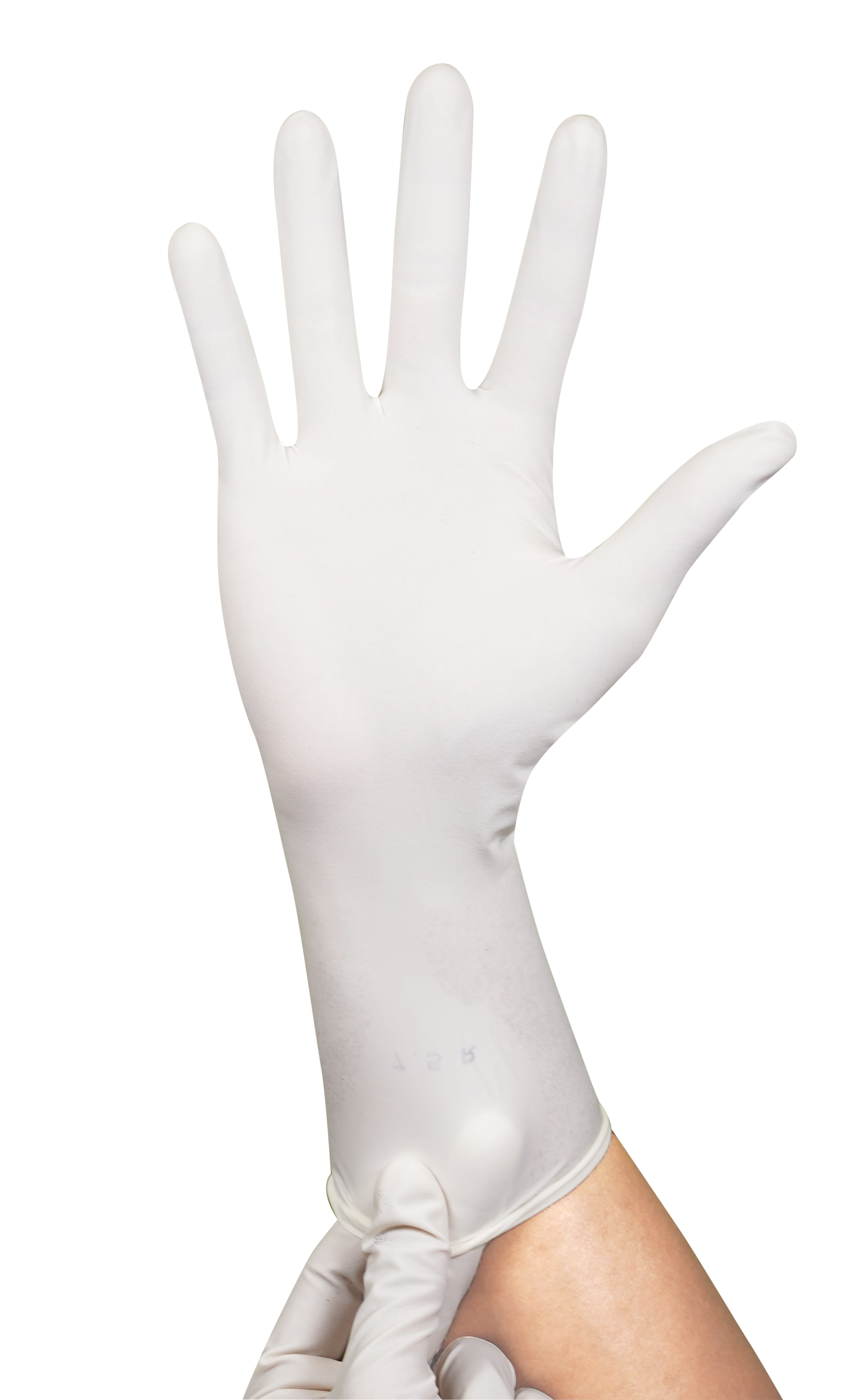
Excellent alternative which eliminates protein allergic reaction and suitable for those who are sensitive to natural rubber latex.
Colour

Synthetic Nitrile
Synthetic Surgical Gloves, Powder Free, Chlorinated
White
Hand specific, curved fingers, palm textured, beaded cuff.
5.5 to 9.0
Yes
The gloves shall maintain their properties when stored in a dry condition. Avoid direct sunlight
The gloves shall have shelf life of 5 years from the date of manufacture
General Surgery

| Size | Length (mm) | Palm width (mm) | Thickness : Single wall (mm) | ||
|---|---|---|---|---|---|
|
|
|
|
||
| Size | Length (mm) | Palm width (mm) | Thickness : Single wall (mm) |
|---|---|---|---|
|
|
|
|
Purpose: To ensure the gloves are free from any chemical substances and safe to use.
| Chemical Test (s) | Test Results (μg/g) |
|---|---|
| Butylated Hydroxyanisole (BHA) | Not Detected |
| Butylated Hydroxytoluene (BHT) | Not Detected |
| Diphenyl Thiourea (DPT) | Not Detected |
| Mercaptobenzothiazole (MBT) | Not Detected |
| Tetramethylthiuram Disulphide (TMTD) | Not Detected |
| Zinc Dibutyldithiocarbamate (ZDBC) | Not Detected |
| Zinc Diethyldithiocarbamate (ZDEC) | Not Detected |
| Zinc Dimethyldithiocarbamate (ZDMC) | Not Detected |
| Zinc Mercaptobenzimidazole (ZMBI) | Not Detected |
| Zinc Mercaptobenzothiazole (ZMBT) | Not Detected |
| Zinc Pentamethyleneditithiocarbamate (ZPMC) | Not Detected |
Remark: Applicable to selected products. Please liaise with our representative for further details.
Reference: ASTM F1671 - Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood - Borne Pathogens Using Phl-X 174 Bacteriophage Penetration as a Test System
Purpose: To test the barrier protection of the gloves from the passages of the viruses.
| Viral Penetration | Test Results |
|---|---|
| PASSES |
Remark: Applicable to selected products. Please liaise with our representative for further details.
To check the presence of bacterial in gloves after sterilization process.
Remark: Applicable to selected products. Please liaise with our representative for further details.
To test the numbers of bacterial living on the glove’s surface that has not been sterilized.
| Bioburden | Test Result |
|---|---|
| PASSES |
Remark: Applicable to selected products. Please liaise with our representative for further details.
To check the presence of bacterial in gloves and it can cause variety of inflammatory responses if enough of their molecules get in the bloodstream.
Remark: Applicable to selected products. Please liaise with our representative for further details.
Reference: ISO 10993-10 (Test for Skin Irritation and Skin Sensitization)
Purpose: Biological evaluation for medical devices for irritation and delayed type hypersensitivity.
| Biocompatibility Test | Test Results |
|---|---|
| Primary Skin Irritation |
PASSES Not a primary skin irritant under condition of the study |
| Skin Sensitization |
PASSES Not a contact sensitizer under condition of the study |
Remark: Applicable to selected product. Please liaise with our representative for further details.