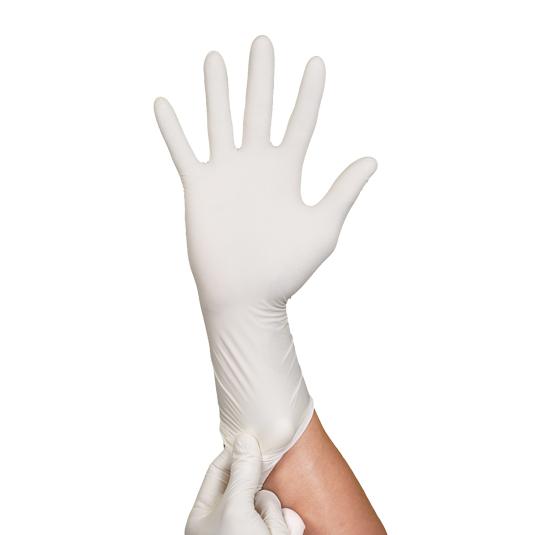
Double Chlorinated Latex Disposable Powder Free Glove
Made from Natural High Grade Rubber Latex which softness provides superior comfort and a natural fit. It provides strong protection from unwanted and dangerous substances with excellent tensile strength and tear resistance. Overall outer surface tackiness selection offer a different grip and handling precision according to different application for Semi Conductor, Electronic, Aerospace and Biotech Industries.
Colour