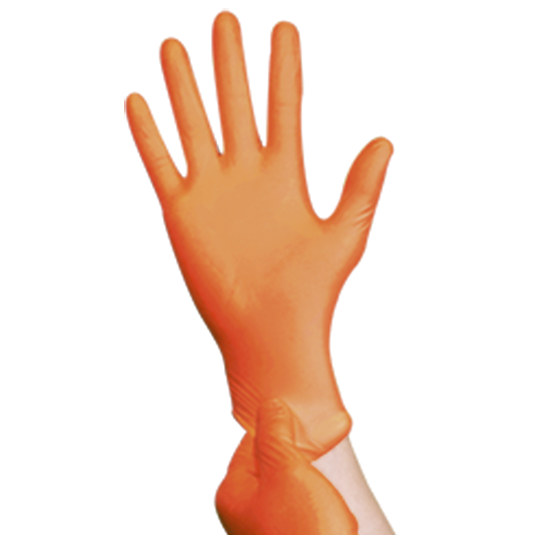
Chemotherapy Nitrile Examination Powder Free Glove
An excellent option for chemotherapy applications where a high level of protection is needed. Available with option for use with 32 Chemotherapy Drugs and Fentanyl. Nitrile Glove is the most popular and reliable glove for medical healthcare and dental industries. Made of premium compounded nitrile eliminating latex allergy concern. It comes with excellent fit, comfort, sensitivity and protection. Its textured surface helps to provide secure grip and added handling precision. Proudly available in an extensive selection of colors giving businesses more options.
Colour














.png)
